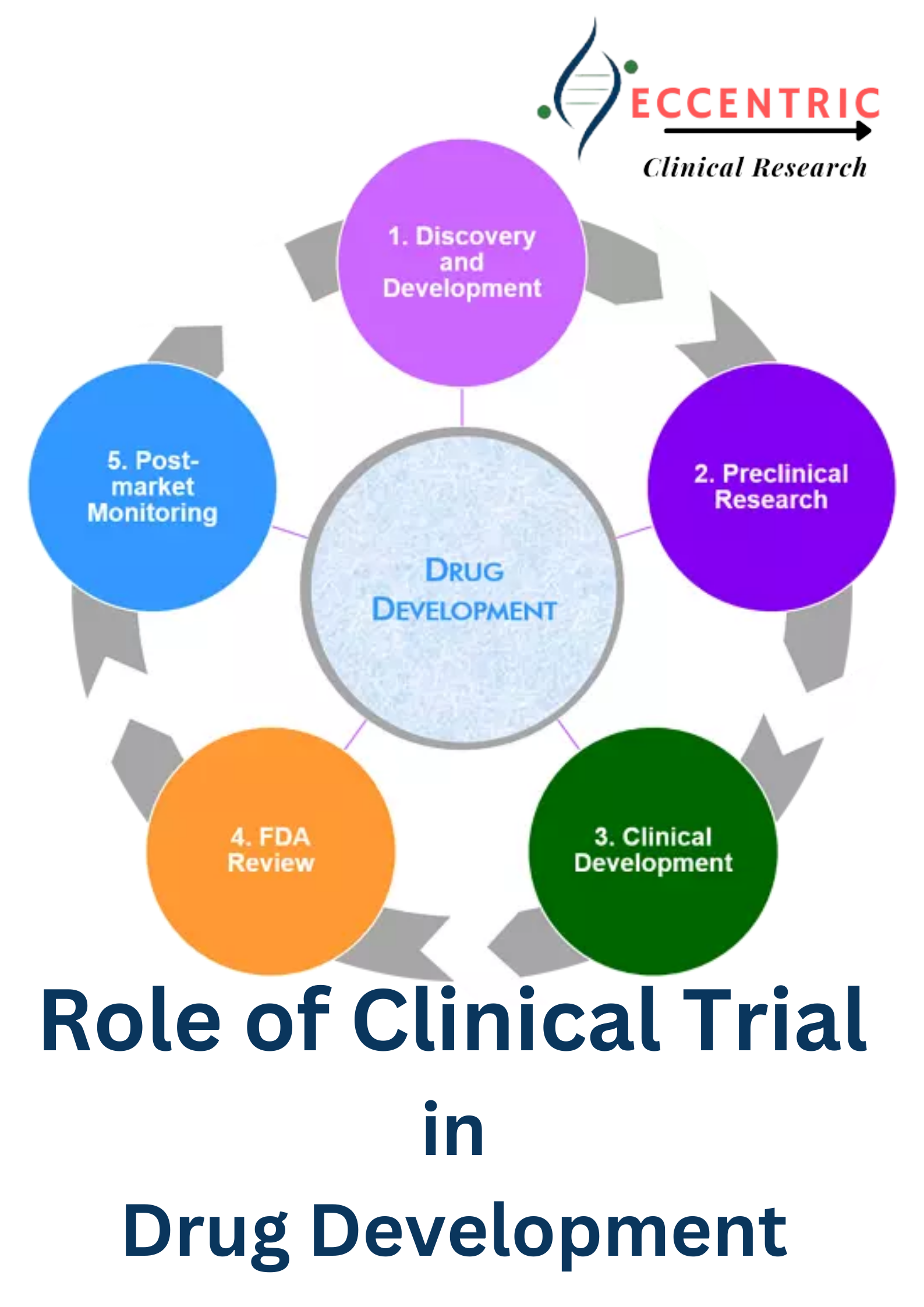
The development of new drug is a complex and carefully regulated process that plays a pivotal role in advancing healthcare and improving patients’ lives. Clinical trials, the critical bridge that transforms a potential drug into a proven treatment. Clinical research studies are carried out on humans with the aim of offering new therapies (drugs and treatments), diagnostic procedures and vaccines.
In this blog post, we will explore how clinical trials help in new drug development, shedding light on the intricate and essential phases of this process.
The proposed clinical study is reviewed ethically prior to the start of trials to ensure the safety of human volunteers. Trials are carried out on drugs to prove consistency and accuracy in the level of safety and effectiveness. Clinical trials are scientific studies designed to evaluate the safety, efficacy, and potential side effects of a new drug. These trials typically involve four key phases:
Phase 1 trial:
The four clinical trial phases after Phase 0, often overlap. Phases 1 to 4 must be completed before granting a license to a new drug Development.
Phase 1 involves:
- Around 20 to 80 volunteers with good health (mostly healthy human volunteers and not diseased individuals, except in rare cases)
- Verification of the most typical side effects of the drug.
- Investigation of the metabolism and excretion of new drug
Phase 2:
Phase 2 trials involve:
- 36 to 300 participants
- Collection of preliminary data on the effectiveness of a drug in treating persons with a specific condition.
- Controlled trials for comparing those using the drug to persons using a different drug or a placebo.
- Continual evaluation of safety.
- Study of side-effects in the short term.
Phase 3 trials:
When Phase 2 trials have confirmed the effectiveness of a drug, the sponsors and REGULATORY AGENCIES will decide on how to conduct big-scale studies in Phase 3 by clinical trial consulting. This includes:
- Around 300 to 3000 participants
- Collection of additional information about effectiveness and safety.
- Studies on varied populations
- Examination of different dosages for determining the ideal amount for a prescription.
- Usage of drug combined with other drugs for determining effectiveness.
Once this phase is completed, total information about the new drug is submitted to Regulatory agencies seeking license to market the Drug for development.
Review meeting
After the REGULATORY AGENCIES approves the product for marketing, there will be studies conducted on its production and post-marketing needs. Such studies are used by regulatory agencies to collect additional optimum use, safety and efficacy information regarding the new drug.
Application of new drug
After the review of the application and prior to Phase 4 trials, the reviewers of the REGULATORY AGENCIES will either issue a response letter or approve the new drug for development. The sponsor of a new drug will finish an NDA (New Drug Application) to request the REGULATORY AGENCIES to approve the marketing of the new drug in the US. The REGULATORY AGENCIES can take up to 60 days to review the file.
After the filing of NDA, the following steps must take place:
- Drug labeling
- Inspection of facility
- Approval of drug.
Phase 4 trials:
Phase 4 trials are conducted after the drug has been approved for marketing. They involve:
- More than 1000 patients
- Comparing and combining with other treatments
- Comprehensive experience in judging the efficacy and safety of a new drug in a huge group of patients.
- Examining side-effects of the drug in the long term.
- Detecting less typical adverse events
- Cost-effectiveness of drug therapy.
How Clinical Trials Drive New Drug Development
Safety and Efficacy Assessment: Clinical trials are a critical testing ground where the safety and efficacy of new drugs are rigorously evaluated. Through careful observation and data collection, researchers can determine whether the drug is safe and effective for its intended use.
Optimal Dosage Determination: The dosing of a new drug is refined through the phases of clinical trials. Researchers aim to find the right balance, ensuring the drug is both effective and safe for patients.
Comparative Studies: Clinical trials often involve comparing the new drug to existing treatments or placebos. This comparative data helps healthcare providers make informed decisions about which treatments to recommend to patients.
Identification of Side Effects: Clinical trials meticulously document side effects and adverse reactions, helping to assess the drug’s safety profile. These findings are critical in understanding the potential risks and benefits.
Patient Selection and Personalization: Clinical trials identify which patients benefit the most from a new drug. This information allows healthcare providers to personalize treatment plans, ensuring that the right patients receive the right medication.
Regulatory Approval: Successful completion of clinical trials is essential for obtaining regulatory approval from agencies like the U.S. Food and Drug Administration (REGULATORY AGENCIES) and the European Medicines Agency (EMA). Approval is the gateway to making the drug available to the public.
Conclusion
Clinical trials represent the heart of new drug development, offering the rigorous testing, data collection, and evaluation needed to bring safe and effective treatments to patients. These trials are the critical pathway for translating scientific discoveries into real-world solutions. While the journey from a potential drug to an approved treatment can be arduous, it is through clinical trials that the promise of new treatments becomes a reality, advancing healthcare, improving patient outcomes, and providing hope for those in need.